
Under normal circumstances, wide variations in salt intake lead to parallel changes in renal salt excretion, such that ECF volume is maintained within narrow limits. Seldin, in Seldin and Giebisch's The Kidney (Fourth Edition), 2008 INTRODUCTIONĮxtracellular fluid (ECF) volume is determined by the balance between sodium intake and renal excretion of sodium. 10.5 schematically illustrates the volume regulatory processes that occur in response to water retention induced by inappropriate antidiuresis.īiff F. For example, a report of body fluid space measurements using isotope dilution techniques in hyponatremic and normonatremic patients with small cell lung carcinoma showed no differences between the two groups with regard to exchangeable sodium space, ECF volume by 35SO 4 distribution, or total body water. 256 Finally, multiple studies of body fluid compartment volumes in hyponatremic patients have not demonstrated either plasma or ECF expansion. Third, results of animal studies in both dogs 255 and rats 256 have indicated that a significant component of chronic hyponatremia is attributable to secondary Na + losses rather than water retention the relative contributions from water retention versus sodium loss vary with the duration and severity of the hyponatremia: Water retention was found to be the major cause of decreased serum in the first 24 hours of induced hyponatremia in rats, but Na + depletion then became the predominant etiologic factor after longer periods (7–14 days) of sustained hyponatremia, particularly at very low (<115 mEq/L) serum levels. Second, an increased incidence of hypertension has never been observed in patients with SIAD, again evidence against significant expansion of the arterial blood volume. First, the concentrations of most blood constituents other than Na + and Cl - are not decreased in patients with SIAD, 254 suggesting that plasma volume is not nearly as expanded as would be predicted simply by the measured decreases in serum. Both experimental and clinical observations are consistent with ECF volume regulation via secondary solute losses.

In most cases of hyponatremia induced by stimulated antidiuresis and water retention, natriuresis also regulates the volumes of the ECF and intravascular spaces. However, volume regulatory processes are not limited to cells. 253 Such coordinate losses of both electrolytes and organic osmolytes from brain cells allow effective regulation of brain volume during chronic hyponatremia.Īlthough recent studies of volume regulation during hyponatremia have focused on the brain, all cells regulate volume by cellular losses of both electrolyte and organic solutes to varying degrees. 252 These losses occur relatively quickly (within 24–48 hours in rats) and can account for as much as one-third of the brain solute losses during hyponatremia. 247 Following the recognition that low molecular weight organic compounds, called organic osmolytes, also constituted a significant osmotic component of a wide variety of cells, studies demonstrated the accumulation of these compounds in response to hyperosmolality in both kidney 248 and brain 249 tissue and conversely that the brain also loses organic osmolytes in addition to electrolytes during volume regulation to hypoosmolar conditions in experimental animals 250,251 and human patients. 245 Whole brain volume regulation via electrolyte losses was first described by Yannet 246 and has long been recognized as the mechanism by which the brain is able to adapt to hyponatremia and limit brain edema to sublethal levels.

An alternative theory is that cell volume is maintained under hypoosmolar conditions by extrusion of intracellular solutes such as potassium. Despite the appeal of this theory, its validity has never been demonstrated conclusively in either human or animal studies. This effect would decrease the intracellular osmolality allowing water to shift back out of the ICF into the ECF, thereby further worsening the dilution-induced hypoosmolality. 227,244 This observation led to the theory of cellular inactivation of solute, which suggested that as ECF osmolality falls, water moves into cells along osmotic gradients, thereby causing the cells to swell at some point during this volume expansion, the cells theoretically osmotically “inactivate” some of their intracellular solutes as a defense mechanism to prevent continued cellular swelling with subsequent detrimental effects on cell function and survival.
EXTRACELLULAR FLUID PLUS
Many past studies have suggested that the combined effects of water retention plus urinary solute excretion cannot adequately explain the degree of plasma hypoosmolality observed in patients.
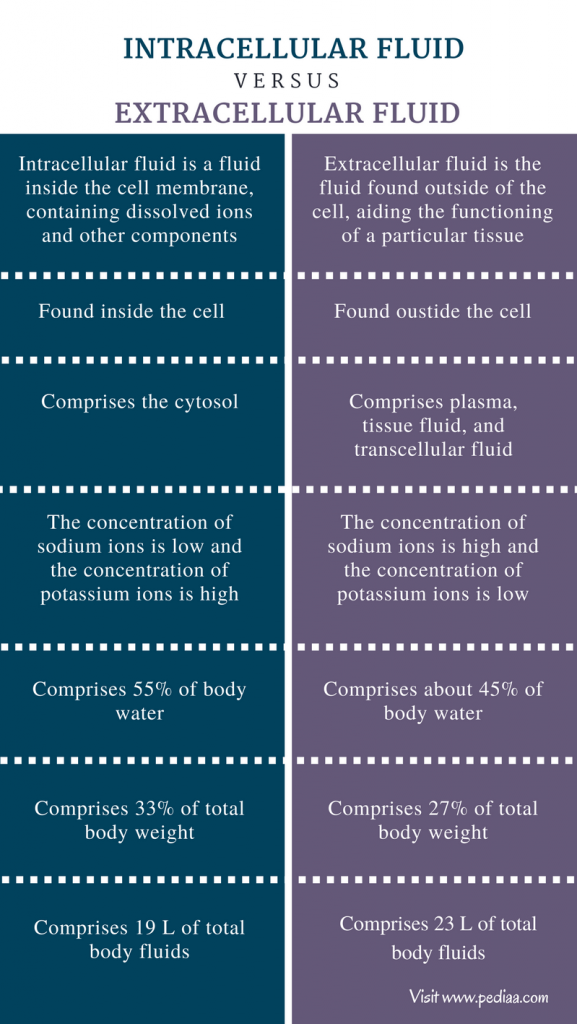
Shlomo Melmed MB ChB, MACP, in Williams Textbook of Endocrinology, 2020 Adaptation to Hyponatremia: ICF and ECF Volume Regulation


 0 kommentar(er)
0 kommentar(er)
